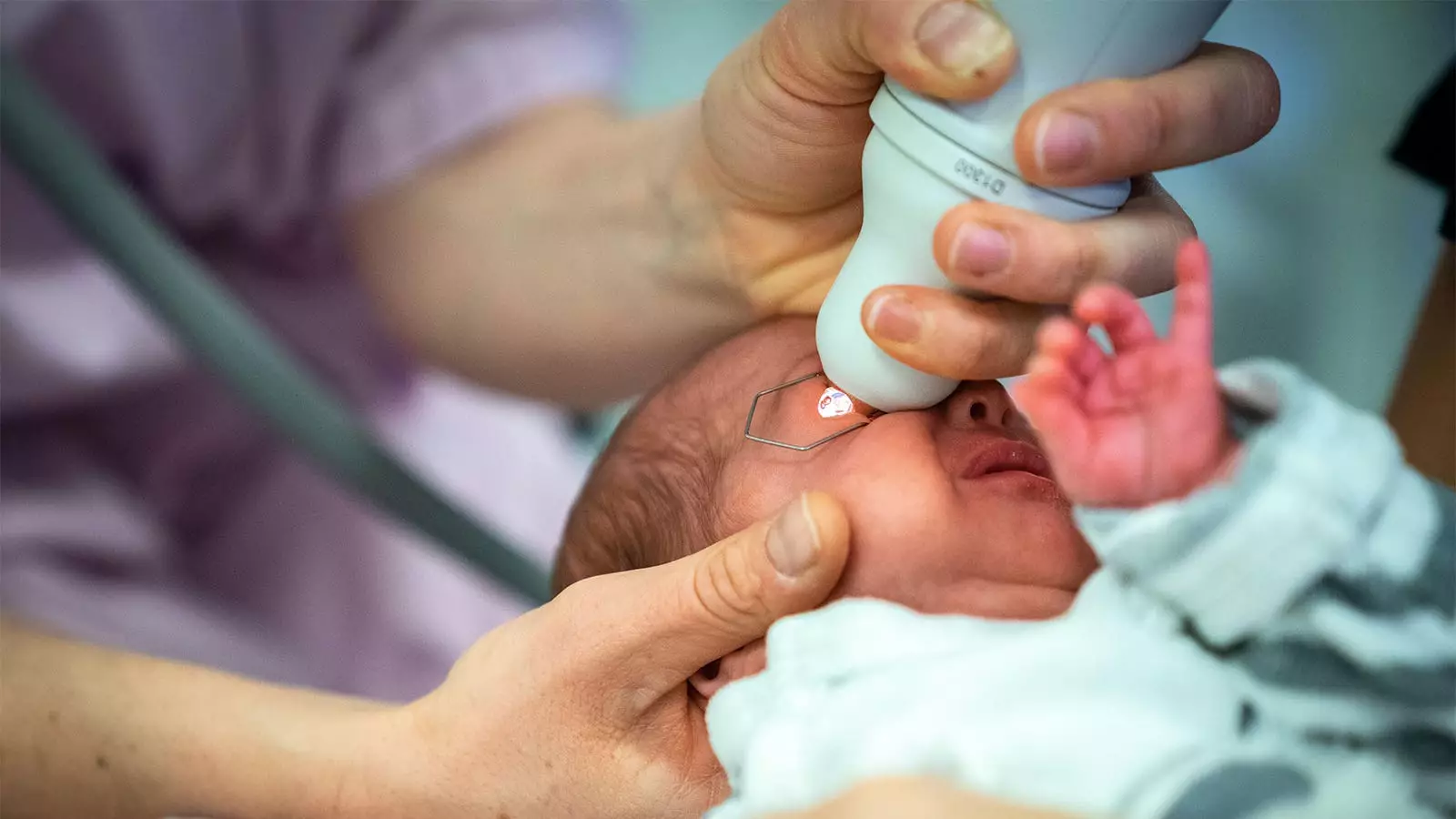
Retinopathy of Prematurity (ROP) presents a significant challenge in neonatal care, as premature infants are particularly susceptible to developing this ocular condition. The traditional approach to screening ROP involves the use of mydriatic drops to dilate the pupils; however, these standard drops have been associated with various adverse effects. In light of these concerns, recent research led by Asimina Mataftsi and her team at Aristotle University of Thessaloniki offers promising findings that could reshape the approach to ROP screening.
The study aimed to evaluate the efficacy and safety of mydriatic microdrops against standard mydriatic drops in a cohort of preterm infants. The trial included 83 infants, all of whom faced the acute challenges posed by ROP screening. It specifically sought to compare the ocular effects of microdrops—a significantly smaller volume—against traditional drops, which typically contain larger volumes and can result in systemic side effects due to their potent formulations. The investigation was crucial as it endeavored to address the delicate balance between achieving sufficient pupil dilation for accurate diagnosis and minimizing the risk of systemic complications potentially triggered by the conventional mydriatics.
Key Findings and Their Implications
The research unveiled that microdrops not only proved to be noninferior but also superior to standard drops in terms of mydriatic efficacy at the 45-minute mark. The measured mean difference was 0.12, with statistical significance marked at P=0.008. At the 90-minute and 120-minute intervals, the microdrops maintained noninferiority, providing further evidence of their effectiveness. These findings are pivotal as they highlight the potential of microdrops to ensure adequate dilation for examination while mitigating the risks associated with larger volumes of standard drops.
In examining the safety profile, the researchers documented a notable drop in oxygen saturation levels among infants treated with standard drops. The mean differences at 45 and 90 minutes were statistically significant, indicating a trend that could be concerning for infants whose physiological conditions may already be precarious. Additionally, the study found that the percentage of hypertensive episodes within 24 hours was relatively lower in the microdrop group compared to the standard group, suggesting that microdrops could diminish the risk of hypertension among this vulnerable population.
The systemic adverse events associated with the conventional mydriatic agents, particularly in a population characterized by extreme fragility, reinforce the need to explore less invasive alternatives. The infants in the study had an average gestational age of 29.7 weeks and an average birth weight of 1,277 grams, making them particularly sensitive to medications. Previous literature has already raised alarms regarding the cardiovascular and gastrointestinal complications linked with standard mydriatics, further prompting the analysis of safer alternatives. Moreover, anecdotal evidence from NICU nurses regarding post-examination complications additions weight to the study’s findings.
Study Limitations and Future Directions
While the results support the efficacy of mydriatic microdrops, the authors cautiously noted certain limitations of the study, most notably the lack of safety outcome data for 30% of the participants. Such gaps in information can pose challenges for wider implementation and acceptance of microdrops in clinical practice. The call for future studies to validate and explore diverse regimens signifies an openness to evolutionary practices within neonatal care.
However, the findings present a compelling case for reevaluating the administration methods during ROP screenings. As the healthcare community continues to prioritize minimizing risk for vulnerable neonates, the adoption of mydriatic microdrops could represent a transformative advancement in the approach to ROP screening.
The investigation by Mataftsi et al. makes a significant contribution to pediatric ophthalmology and neonatal care, offering tangible evidence for a paradigm shift in mydriatic use. By highlighting the dual advantages of enhanced efficacy and reduced systemic risks, the findings advocate for the broader adoption of microdrops in ROP screening, ultimately ensuring better outcomes for our most fragile patients. Future explorations may help confirm these findings and extend their implications across various populations, solidifying a more secure path for managing retinopathy of prematurity.


Leave a Reply