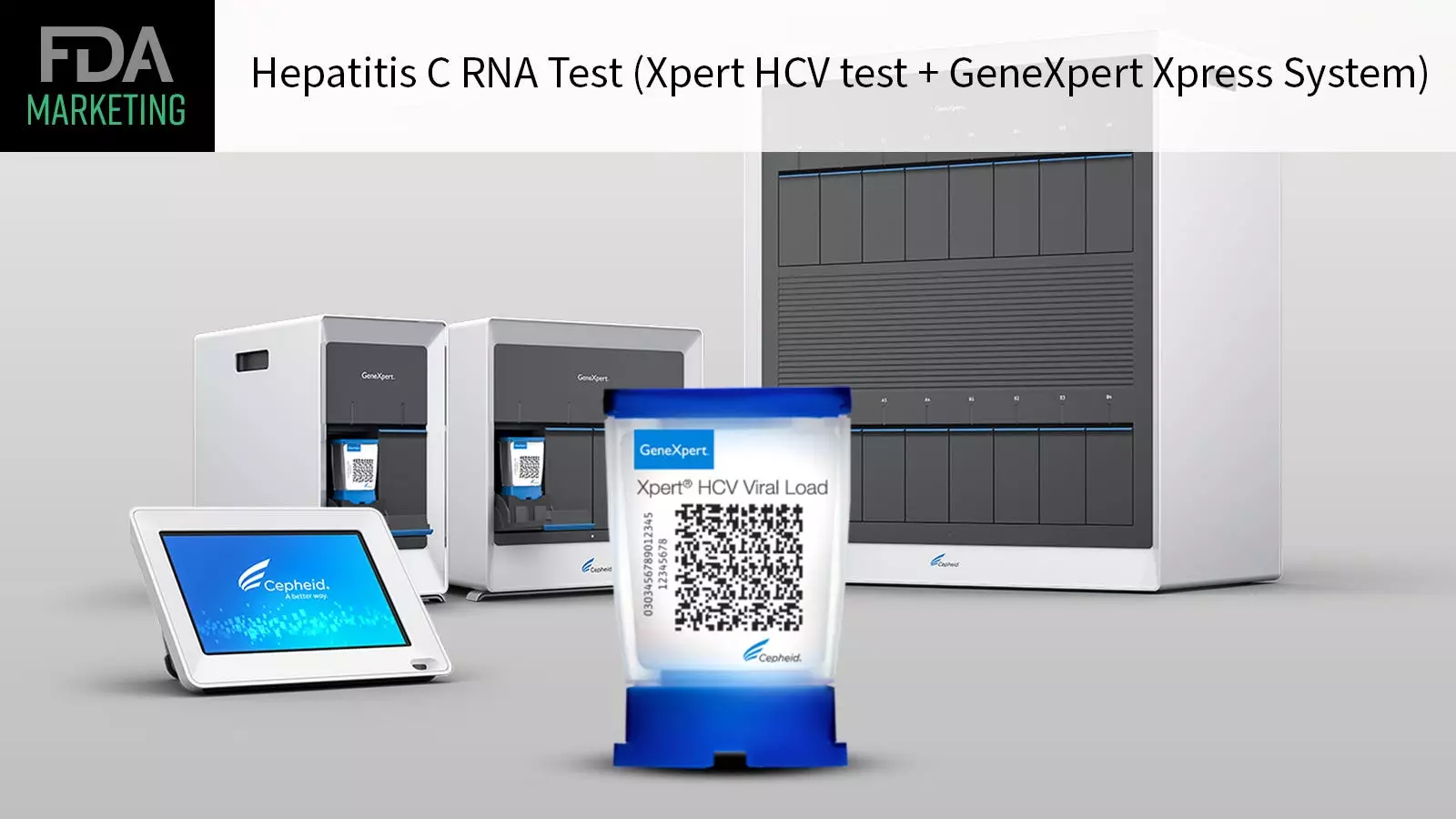
The recent FDA approval of the first point-of-care test for hepatitis C virus (HCV) marks a significant milestone in the fight against this potentially deadly infection. This innovative test, known as the Xpert HCV test and GeneXpert Xpress System, allows at-risk adults to be rapidly diagnosed and linked to care if necessary. Unlike traditional tests that required samples to be sent to a central lab, this new test can be used in various healthcare settings, including doctors’ offices, urgent care centers, and emergency rooms, thanks to its Clinical Laboratory Improvement Amendments (CLIA) Certificate of Waiver. The ability to deliver results within an hour using a fingertip blood sample means that providers can quickly identify cases of hepatitis C and discuss treatment options with patients during the same visit.
The lack of convenient and widespread testing options has contributed to many people being unaware of their hepatitis C status. The availability of the new point-of-care test aims to bridge this gap by empowering healthcare providers to diagnose and treat patients promptly. According to Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, equipping providers with tools for same-day diagnosis and treatment could lead to hundreds of thousands more hepatitis C patients being identified and treated, ultimately preventing disease progression and further spread of the virus.
Challenges and Opportunities in Hepatitis C Management
While effective oral cures for hepatitis C have been available for years, cure rates in the U.S. remain low, highlighting the need for improved testing and diagnosis strategies. The CDC’s recommendation for universal HCV screening underscores the importance of early detection and treatment to prevent complications such as liver cancer or liver failure. With as many as 4 million Americans estimated to have hepatitis C, the implications of undiagnosed and untreated infections are significant. The availability of the new point-of-care test provides hope for more people to be cured, but its success hinges on factors such as affordability and accessibility.
The FDA’s approval of the first point-of-care test for hepatitis C virus represents a critical step forward in the management of this infectious disease. By streamlining the testing process and enabling rapid diagnosis and linkage to care, this new test has the potential to transform the landscape of hepatitis C diagnosis and treatment. However, it is crucial to address challenges such as false-positive and false-negative results to ensure the test’s accuracy and reliability. As efforts continue to expand access to testing and treatment for hepatitis C, the ultimate goal is to reduce the burden of this disease and improve outcomes for affected individuals.


Leave a Reply